
Thermal decomposition : At high temperatures (above 900☌ or 1652☏), carbon tetrachloride can undergo thermal decomposition.This inertness makes it useful in certain applications, such as as a solvent for reactions that need an inert environment. It is not easily involved in typical organic reactions, such as nucleophilic or electrophilic substitutions. Inertness : Carbon tetrachloride is considered an inert compound in many chemical reactions.Due to its nonpolar nature, it is not a good solvent for polar or ionic compounds. It can dissolve oils, fats, resins, waxes, and many other nonpolar or weakly polar substances. Solvent properties : Carbon tetrachloride is an effective solvent for a wide range of organic compounds.However, it can react with strong reducing agents or reactive metals under certain conditions. It is non-reactive with most common substances, such as water, acids, and bases. Stability : Carbon tetrachloride is a stable compound under normal conditions.Vapour pressure: 11.7 mmHg at 25 ☌ (77 ☏)Ĭhemical Properties of Carbon Tetrachloride Formula.Flashpoint : Non-flammable, but it can ignite at high temperatures or in the presence of an open flame.Solubility : Carbon tetrachloride is relatively insoluble in water but dissolves in many organic solvents, such as ethanol, acetone, and benzene.
Here are the physical properties of carbon tetrachloride: Physical Properties of Carbon Tetrachloride FormulaĬarbon tetrachloride (CCl 4 ) is a colorless liquid compound that is also known as tetrachloromethane.
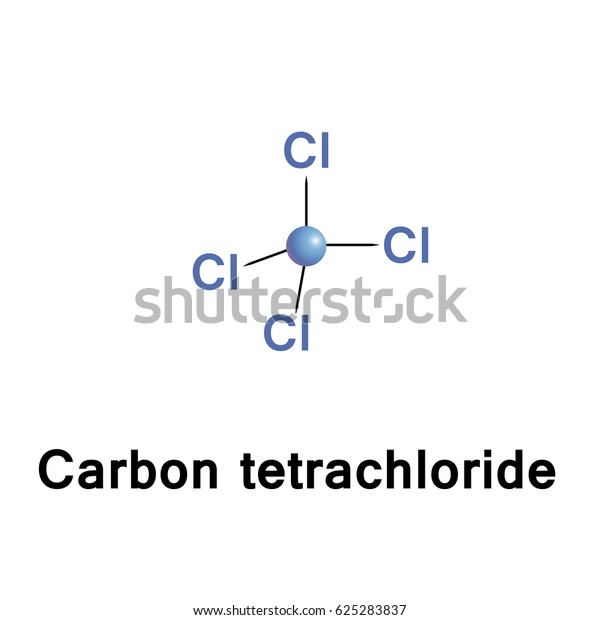
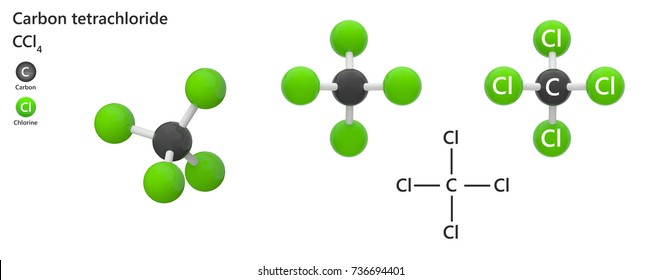
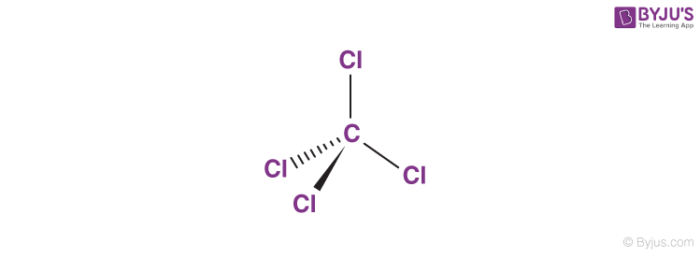
Fire Extinguisher: Carbon tetrachloride was utilized in fire extinguishers as a liquid that could be sprayed onto fires.It was particularly useful in the dry cleaning industry for removing stains from fabrics. Solvent: Carbon tetrachloride was commonly used as a solvent for various organic compounds, such as oils, fats, resins, and waxes.Structural Formula of Carbon TetrachlorideĬarbon tetrachloride is composed of one carbon atom bonded to four chlorine atoms, arranged in a tetrahedral shape. However, it is known to be toxic and has been phased out in many countries due to its harmful effects on human health and the environment. It is also known by the common name “tetrachloromethane.”Ĭarbon tetrachloride is a stable compound and is mainly used as a solvent, particularly in industrial applications. The o of the mono- and the a of hepta- are dropped from the name when paired with oxide.Carbon tetrachloride, with the chemical formula CCl 4, is a colourless, heavy, nonflammable liquid compound. \) emphasizes that the formulas for molecular compounds are not reduced to their lowest ratios.


 0 kommentar(er)
0 kommentar(er)
